|
|
| [Home] [WTC (demolition)] [Traces of thermate at the WTC] [Sulfur at the WTC] |
|
The story... Sulfur discovered on metal samples at the WTC may indicate the use of thermate. Professor Steven Jones justifies this claim by first quoting FEMA, who discovered steel that had been affected by sulfur : “Evidence of a severe high temperature corrosion attack on the steel, including oxidation and sulfidation with subsequent intergranular melting, was readily visible…The severe corrosion and subsequent erosion of Samples 1 and 2 are a very unusual event. No clear explanation for the source of the sulfur has been identified.” He points out that "sulfur is used with thermite (called “thermate”) to cut fast through steel", and "aluminum and sulfur" (amongst other “unusual” chemicals) are also present in samples of WTC metal that Jones has tested (source). Thermate, he proposes, would explain all of these issues at once: but is that really true? ...to melt steel would require STEEL (not air) temperature of over 2,700 degrees F... Which would be no problem for thermite, let alone thermate, as a source used by Professor Jones confirms: In recent years the use of thermite reactions as incendiary devices has gained popularity with arsonists because they are easily ignited with a match, burn quickly and can generate a very intense heat in excess of 4000 degrees F. So what temperature did the FEMA steel actually reach? Here’s the information Professor Jones left out: Sample 1: Further studies saw the minimum temperature required to produce the effects reduced still further (our emphasis): The as-fabricated microstructure consisted of a hot worked banded structure of ferrite and pearlite. In severely "eroded" regions where the thickness had been reduced to less than a 1/16 of and inch significant decarburation was observed. In addition, some pearlite bands presented regions that had re-austentized as well as regions where the pearlite had started to spheroidize. These observations indicate that steel had experienced temperature between 550 and 850 degrees C. Even if the highest quoted temperatures are the most accurate, they are still within the range that can be produced by nothing more than a fire. BRE (Building Research Establishment) carried out a project based around "the development and validation of a CFD-based engineering methodology for evaluating thermal action on steel and composite structures" a few years ago. They build a fire compartment, used various loads (either wood, or wood with plastic) and reported peak temperatures: As can be seen in the above table, peak measured temperatures exceeded 1300°C in five tests, this measurement being supported by the observation of total heat fluxes of up to 350 kW/m2 and velocities of over 15m/s. Ordinary fuels with a little plastic, and the right conditions, yielded high temperatures. And this applied even to the steel itself, where the maximum temperature record in four tests proved to be 1220, 1301, 1245 and 1196 °C (that’s a peak of 2372 °F). (More on the temperatures achievable by fires on this page.) Thermate is a high-level thermite analog containing sulfur developed by the military (see http://www.dodtechmatch.com/DOD/Patent/PatentDetail.aspx?type=description&id=6766744&HL=ON). Thermate combines aluminum/iron oxide (thermite) with barium nitrate (29%) and sulfur (typically 2% although more sulfur could be added). The thermate reaction proceeds rapidly and is much faster than thermite in degrading steel leading to structural failure. Thus, both the unusually high temperatures and the extraordinary observation of steel-sulfidation (Barnett, 2001) can be accounted for -- if the use of thermate is allowed in the discussion. Note that other oxidizers (like KMnO4) and metals (like titanium and silicon) are commonly used in thermite analogs. Sulfur could be 2% of the thermate, then, or perhaps more. And how much thermate might be required? Professor Jones estimates the total based on another controlled demolition here: Phone interview with demolition expert, Brent Blanchard, 10 Feb 2006... Two percent of 1,000 pounds gives us 20 pounds of sulfur, then, 40 for both towers, let’s say 60 pounds if we allocate the same to WTC7. How does that compare to other possible sources?
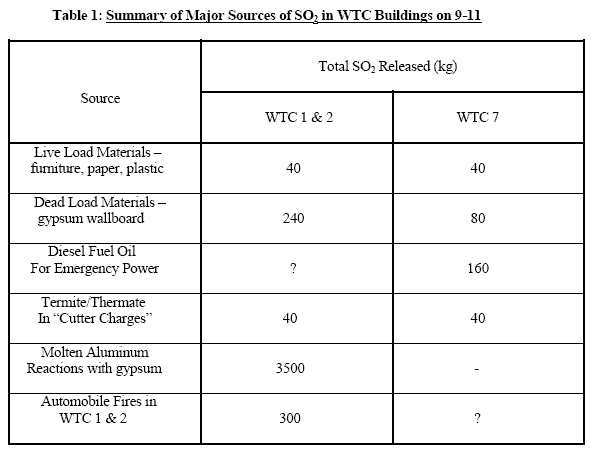
This table, from Frank Greening’s “Sulfur and the World Trade Disaster”, offers some clues. Note that this doesn’t translate directly to our figures: Greening is talking about SO2 rather than sulfur (that’s released from the sulfur when the reaction starts, and what attacks the steel), for example. He’s also assuming 2000kg of thermate would be used per WTC building, 4409 pounds, more than four times Professor Jones’ estimate. But even with Greening’s more generous quantities, the point is clear. There’s far more SO2 available from other sources then thermite, 4360kg to 80kg on this estimate, and that’s only considering some of the major sources. Some of these are more speculative then others, so please read the whole paper to assess them for yourself, but even if we throw away the 3,500kg contribution from “Molten aluminum reactions with gypsum”, for instance, that still leaves 860kg from elsewhere. And it’s not only Greening who has alternative suggestions for sulfur sources. George Vander Voort, who has worked on analysing the steel samples, asks the following questions at the end of a presentation on the topic: Could the source of the sulfur be as simple as - -“acid rain” And a National Science Foundation study suggests gypsum may be an adequate explanation for high levels of sulfur and other chemicals they found in New York harbour sediment: The high levels of calcium, strontium, and sulfur concentrations found in the near-surface sediments of the cores, are consistent with presence of gypsum as a parent material. Gypsum is extensively used as drywall in building construction. We have FEMA metal samples that have seen temperatures within the possible range of a fire, then, and traces of sulfur that could have come from a wide variety of sources. What we don’t have is any explanation of why thermate is a more likely source than anything else, and until that arrives the sulfur does not appear to be a significant indicator of anything at all. |
| [Home] [Hijackers] [Foreknowledge] [Stand down] [WTC (demolition)] [WTC (other)] [WTC7 and Silverstein] [Pentagon] [Flight 93] [bin Ladin] [Obstructing Justice] [Afghanistan] [Others] [Investigations, more] [What's New?] |